

A common example you've experienced is getting a shock when you touch something metal, usually in the winter when the air is dry.
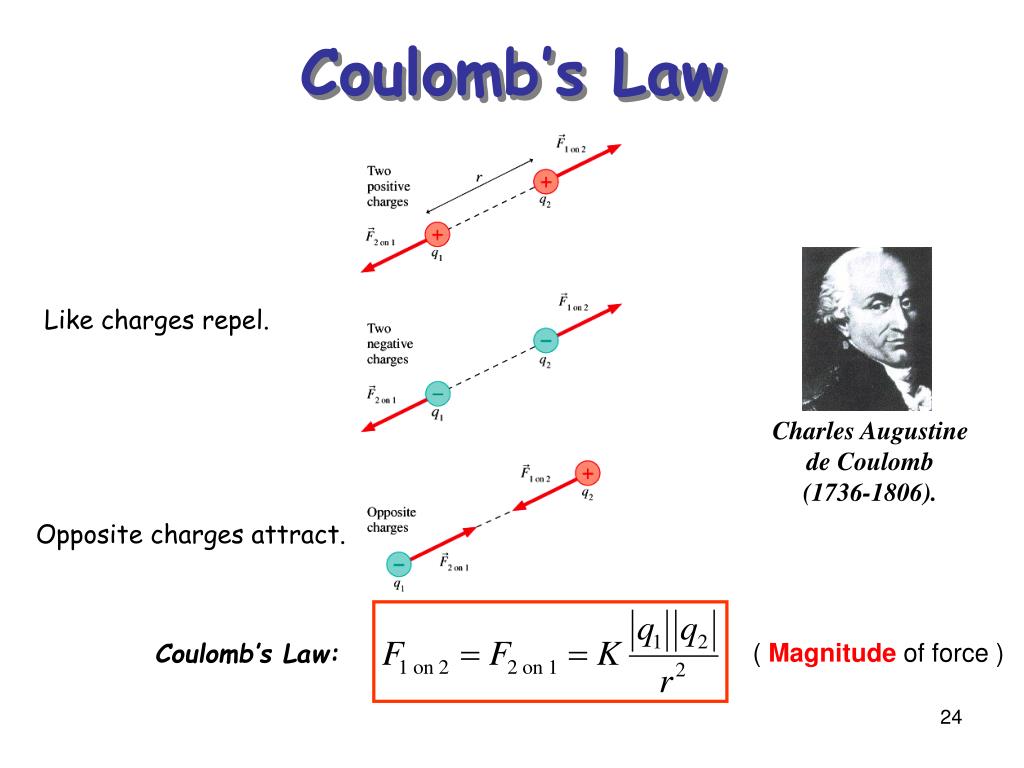

Static electricity refers to charge that doesn't move unless "triggered" to do so. As the flow-chart above shows, we can divide our discussion about charge into static electricity phenomena and electric current. In the following sections we'll figure out the details of how charges interact and the units of charge. How many times have you been walking down the street and gotten ejected from Earth by gravity? Gravity has no repulsive component it is a purely attractive force. Forces between charges can be attractive or repulsive. This ought to make you pause for a second because it is already vastly different than the gravitational force, our other invisible force. Those always tend to pair up evenly.Ĭharges exert invisible forces on one another in a specific and predictable way. The universe also tends to balance charges in a given system (a defined piece of the universe), there are generally the same number of positive and negative charges.įor example, in a salt crystal consisting of positively charged sodium ions (Na +) and negatively-charged chloride ions (Cl -), it is highly unlikely that we will have an unpaired charge. When the charge of something changes, it's because it loses or gains a negatively-charged electron. In normal processes (things we would encounter in day-to-day life) charges are neither created from nothing nor destroyed. Objects that are neutral may be that way because of their inherent nature, like the neutron, but more commonly they are neutral because they contain equal numbers of negatively-charged particles (electrons) and positively charged ones (protons).Ĭharge is also a conserved quantity. Matter may be positively-charged, negatively-charged or neutral (not charged). We now know those to be negatively-charged electrons. In more controlled experiments, we can observe that charged objects exert invisible forces on one another.īenjamin Franklin, an early researcher in electricity and charge, assigned the label positive to the charges that tend to move the most. We understand charge because we can observe lightning, see sparking between wires and we can get an electric shock when things like fabrics rub together in the dry air of winter. 'Charge ordering' is not a unique feature of Coulombic interactions as is sometimes supposed, but will occur whenever the potential between unlike species is strongly attractive.Electric charge, like mass, is a fundamental property of matter. In particular, the distribution functions for the screened Coulomb model exhibit 'charge ordering' g ++(r) oscillates out of phase with g +-(r) and the first peak in S ++(k) lines up with the first minimum in S +-(k). Their calculations, which are based on the mean spherical approximation and on the hypernetted-chain approximation, demonstrate that very short-ranged screened potentials can produce g alpha beta (r) and S alpha beta (k) which are very close to those obtained for the conventional restricted primitive model of an alkali halide. In the second the potentials are assumed to be screened Coulomb outside the core. In the first model the interionic potentials are taken to be Coulombic outside the hard core this is the conventional restricted primitive model. The authors have calculated the radial distribution functions g alpha beta (r) and the partial structure factors S alpha beta (k) for two different charged hard-sphere models of a molten alkali halide.


 0 kommentar(er)
0 kommentar(er)
